MFT Gateway is a hosted Software as a Service (SaaS) solution that enables file exchange over the AS2 or SFTP protocol, without the need to install or maintain.
- Blog
- FDA submission view in MFT Gateway
AS2 | MFT
FDA submission view in MFT Gateway
FDA Submission View in MFT Gateway streamlines regulatory compliance, ensuring secure electronic submissions via the FDA's Electronic Submission Gateway (ESG).

Lahiru Ananda
Published: 24 Jul 2024

The FDA Submission View in MFT Gateway facilitates regulatory compliance by streamlining electronic submissions via the FDA’s Electronic Submission Gateway (ESG), ensuring secure and efficient data transmission.
FDA and FDA Submissions
What is the FDA?
The Food and Drug Administration (FDA) of the United States functions as a federal organization under the Health and Human Services department for the sole purpose of upholding this institution which takes care of citizen’s well-being thus guaranteeing their safety as far as medicinal purposes are concerned. Its other mandate includes monitoring cosmetics manufacture together with dietary products; establishing rules governing tobacco use as well as electrical appliances that emit waves or rays.
FDA submissions
The FDA submission process involves regulatory documents sent electronically to the FDA, primarily through the Electronic Submission Gateway (ESG). ESG enables submissions that are secure, efficient, and compliant, enabling companies to meet regulatory requirements and allowing the FDA to safeguard public health by assuring product safety and efficacy.
Initially, companies design their submission packages in accordance with FDA guidelines and ensure that all the necessary documents are formatted for regulatory compliance. Using secure protocols such as Applicability Statement 2 (AS2), they have to submit these documents when they are fulfilled. At this stage MFT Gateway can help in encrypting your submissions and transfer them to FDA’s ESG securely via AS2 protocol after encrypting and signing them digitally.
Once you have made your submission, Electronic Submission Gateway (ESG) will send back acknowledgement receipts thereby confirming it was received successfully as well as assign a tracking number unique only to this particular file. FDA goes ahead to inspect applications made in order to ascertain that they conform to certain medical rules such as being safe or working correctly within legal limits and guidelines.
FDA Submissions with MFT Gateway
When you finish preparing your FDA submissions, the MFT Gateway will take care of the secure submission process and handle acknowledgments received from the FDA through the AS2 protocol. Below are the basic steps that you will need to adhere to in order to submit your content to the FDA using MFT Gateway:
Set Up an AS2 Station: Configure an AS2 station for your organization. This station will be used to send and receive AS2 messages. Once you created the station you need to share the station’s partner configuration information (which you can directly share through an email from the partner configuration window) with FDA.
Configure an AS2 Partner: Set up an AS2 partner representing the FDA. This configuration will allow you to securely exchange messages with the FDA. The FDA will share with you the required information and based on that you need to configure an AS2 partner representing the FDA.
Configuring FDA specific HTTP headers
When submitting messages to the FDA via AS2, certain custom HTTP headers are typically required to ensure the submission is processed correctly. These headers help in identifying and handling the transmission properly. You can easily add these specific transport headers to your FDA partner with custom header profile functionality.
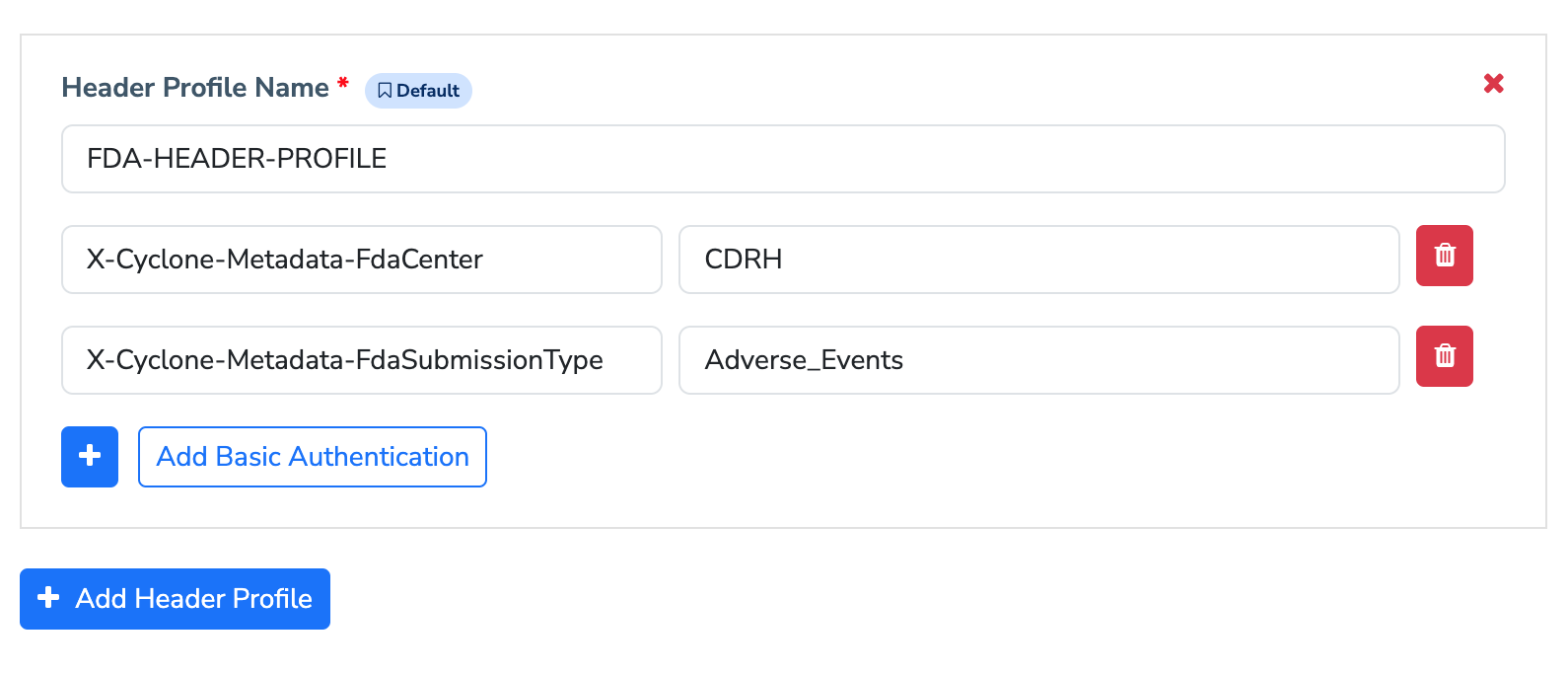
Submitting message to FDA
The MFT Gateway message submission view is an easy and convenient way to submit to the FDA with a few clicks. By default, the send message option will pick FDA pre-configured default header profile. However, from the dropdown menu, you may select any header profile that you have configured for your preferred FDA partner.
After you select the necessary details (i.e.: Message Sender and Message Receiver) and have uploaded the content you required to submit from the file uploader, all you have to do is click the send button. Based on your details, the MFT Gateway submits your content to the FDA securely through AS2.
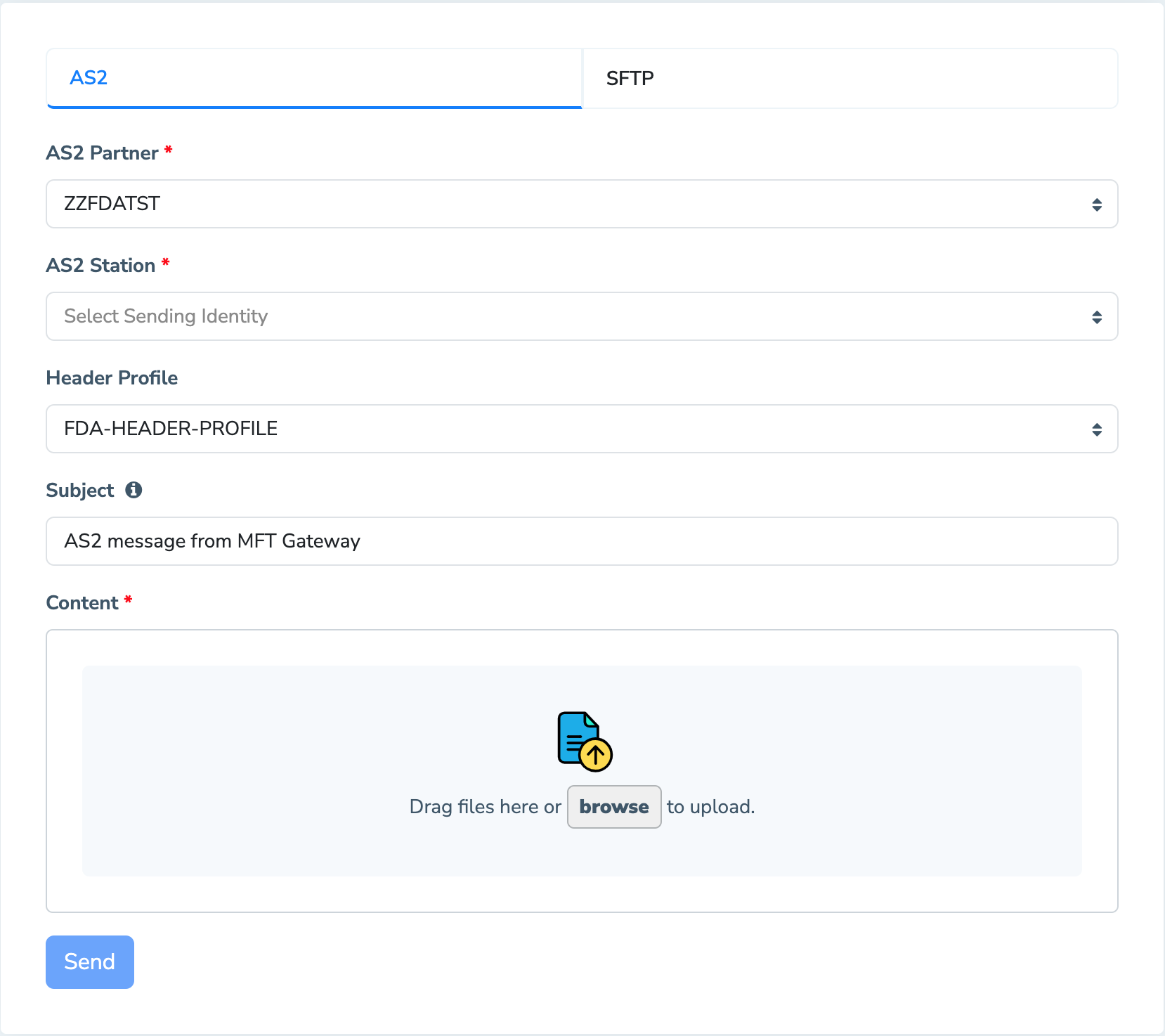
Automating the flow of message delivery can also be achieved with any of the integration methods provided by the MFT Gateway as well.
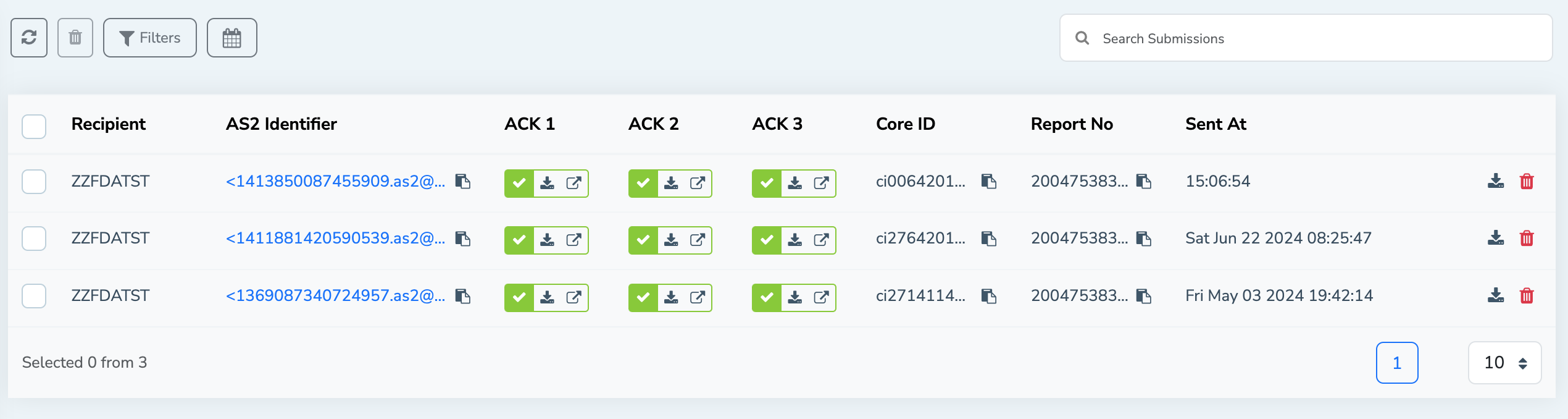
FDA Submissions list view
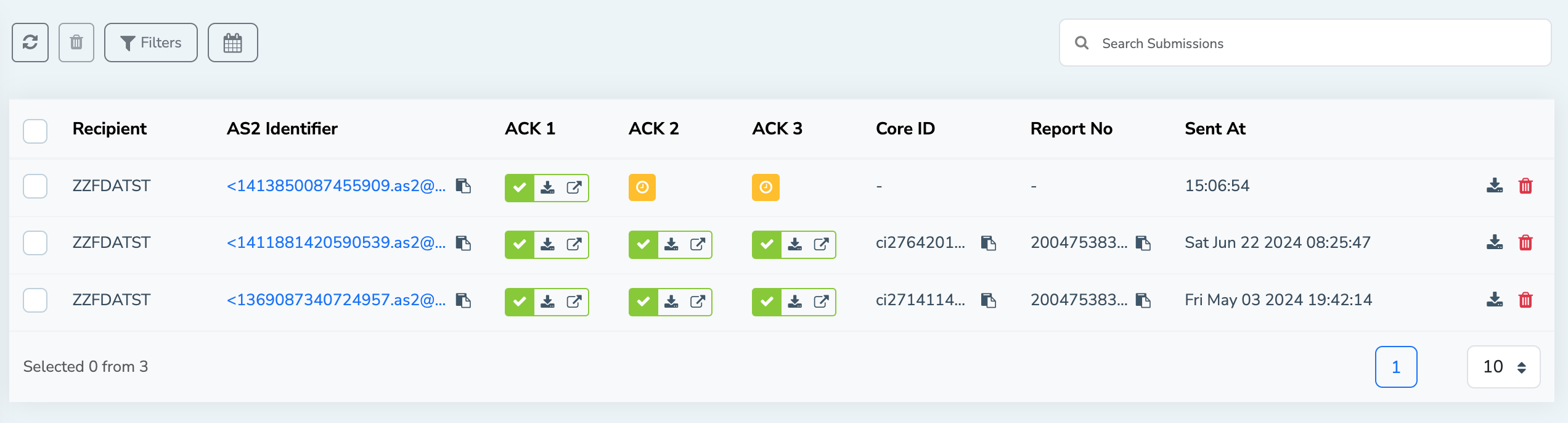
Once the data you sent through the MFT Gateway is submitted successfully to the FDA, submission information can be viewed on the FDA submissions list view. From the FDA submissions view, you can find the following information associated with the submission.
- Message recipient
- AS2 identifier
- Acknowledgements status
- Core Identifier
- Report number
- Timestamp
This allows you to easily track and manage your FDA submissions from one single location.
FDA Acknowledgements
FDA acknowledgments are automated responses sent by the FDA’s Electronic Submission Gateway (ESG) to inform the recipient about the status of regulatory submissions that the sender has made. These acknowledgments play a role in enabling submitters to establish that their submissions were received and are now being acted upon. Usually, FDA acknowledgments may appear in three categories:
Acknowledgment 1 (ACK 1): Receipt Acknowledgment:
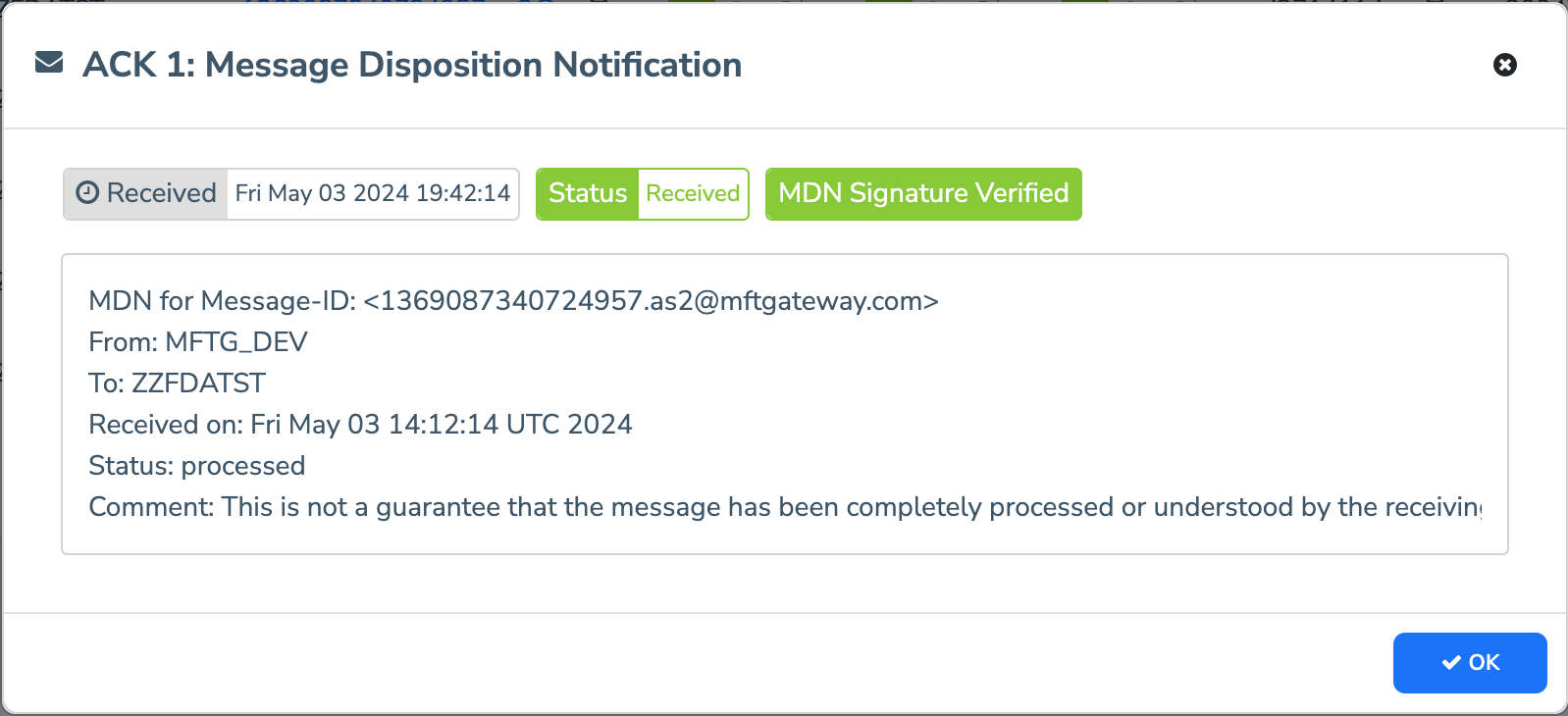
This is the first confirmation that the submission has been received by the ESG. It acknowledges a successful file transfer and known presence of the package in the FDA system.
Usually, when transmission is done through AS2, the MDN (Message Disposition Notification) is considered as the ACK 1. The MFT Gateway will receive the ACK1 (MDN) synchronously and you will be able to see ACK1 information immediately with the submission entry.
Acknowledgment 2 (ACK 2): Validation Acknowledgment:
This acknowledgment confirms that the submission has been preliminarily validated, indicating that the submission package has been properly formatted and complies with the FDA’s basic technical requirements. Otherwise, ACK 2 will provide an error message specifying what needs to be corrected.
When FDA sends the ACK2 (Usually as a separate incoming AS2 message) the MFT gateway will identify the correlation between the original submission and automatically update the ACK2 information in the FDA submissions view.
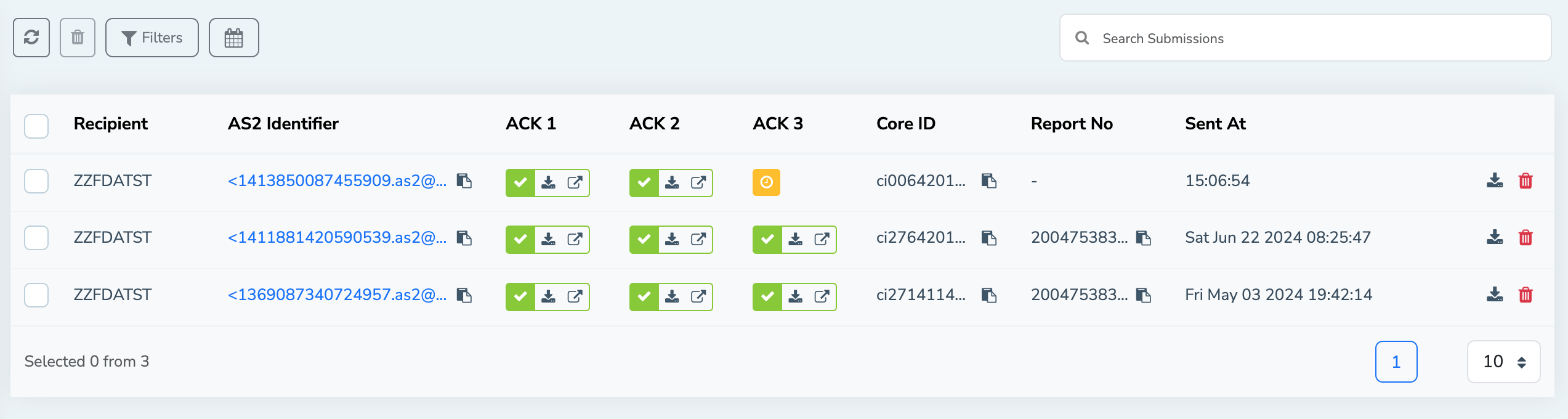
Acknowledgment 3 (ACK 3): Center Acknowledgment:
ACK 3 is the final acknowledgement received from the FDA for a particular submission. This acknowledgement indicates that the submission has been successfully delivered to the right FDA Center for further review. Also, it indicates that the regulatory review process has officially started.
As with ACK 2, the MFT Gateway will update ACK 3 information in corresponding submission when a new AS2 message is received from the FDA with ACK 3 information.
From the FDA submission view, you can easily navigate and see more information about the corresponding acknowledgement using the open ACK button. Also, you can directly download the content of a particular acknowledgement from the list view without navigating to the actual AS2 message as well.
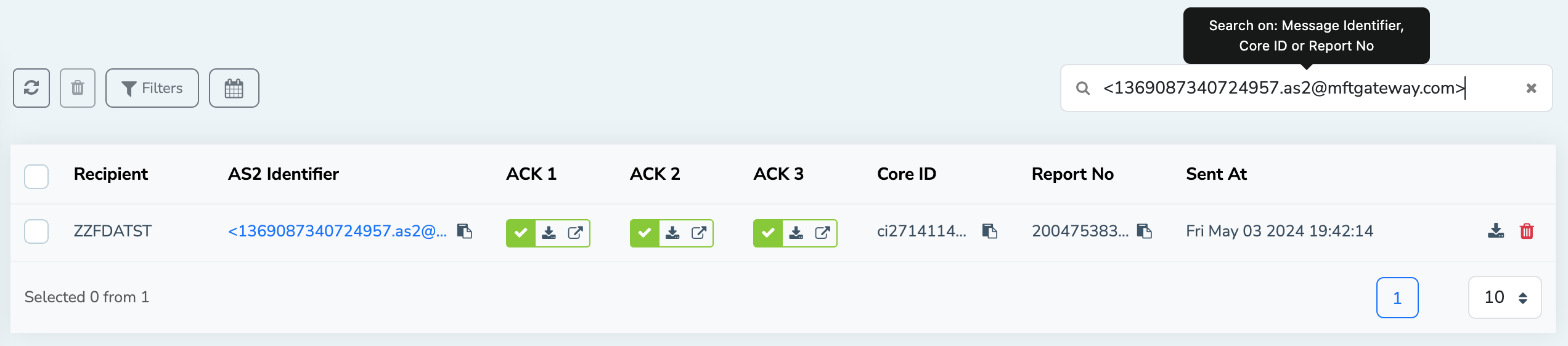
Filtering FDA Acknowledgements
The MFT Gateway provides you the ability to filter FDA submissions based on the sending station and receiving partner identifiers. Also, you can use filtration based on relative and absolute timestamps as well. Furthermore, the MFT Gateway allows you to filter specific submissions based on the AS2 message identifier, Core Identifier or Report number as well.
Get started with MFT Gateway
Check out MFT Gateway, to ease and streamline your FDA ESG submissions; start a free trial with no strings attached.
Feel free to reach us anytime for any questions, doubts or guidance along the way.

Talk to an EDI Expert
Join hundreds of organizations already taking full control of their B2B AS2 communications with our trusted solutions. Contact us today to tailor a solution that fits your specific AS2 EDI needs.
Related Articles
View All BlogsExplore our product stack
Try before you buy with a 30-day Free Trial
No commitment, all value. Try the AS2 Solution Risk-Free and discover how our solutions can transform your business workflows. No credit card required.
Explore Your Possibilities
Elevate AS2 Communications with our EDI and AS2 Solutions
See how our AS2 and EDI solutions can simplify your integrations, boost efficiency, and keep you compliant—request a personalized demo today.