MFT Gateway is a hosted Software as a Service (SaaS) solution that enables file exchange over the AS2 or SFTP protocol, without the need to install or maintain.
- Blog
- How to create an FDA compatible submission archive
FDA
How to create an FDA compatible submission archive
This article discusses how to create a proper submission archive for the FDA

Udith Gunaratna
Published: 27 Jun 2024

Making regulatory submissions to FDA (US Food and Drug Administration) includes a number of steps. One crucial step is to ensure that the final submission archive is in the expected format and uses the correct encodings and schemes.
In this article, we will discuss how to use some popular archival utilities to create an FDA-compatible archive.
This article focuses solely on the steps for creating the archive and assumes you already have the necessary content prepared in the correct file structure for submission to the FDA.
Basic requirements
First, let’s examine the basic requirements that a submission archive must meet to be accepted by the FDA systems for processing.
- Submission archives should be in the
tar.gz(tarred and then gzipped) format. - Archives need to be well-formed, such as by having parent directory entries ahead of each file; for example, if a
tarfile has a
foo/bar/baz/data.xml, it should be preceded by afoo/bar/baz/directory entry, which should in turn be preceded by afoo/bar/, and so forth. - Archives should use GNU filename encoding, and STAR scheme for storing large file entries.
Now, let’s explore how we can use some widely used archival utilities to create an archive that meets these requirements.
In Microsoft Windows
In MS Windows, we will use the popular file archival utility named 7-Zip for this purpose. You can download this utility from their official website and install it on your Windows machine.
Creating a .tar.gz file in 7-Zip is a two-step process. First, we need to create a .tar file with the necessary content.
Then, we create a .tar.gz file by gzipping that tar file.
Creating a tar file with submission content
- Open the previously installed 7-Zip application and a file browser window will come up.
- Navigate to the location where your submission-ready content is available and select the folder which should be included in the archive (generally this will be the folder with Application ID as the name).
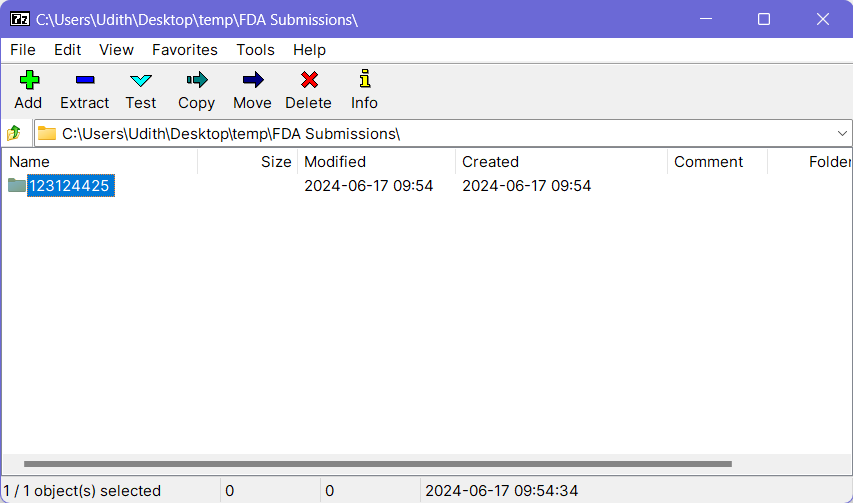
- Click the Add button at the top-left corner and it will open the “Archive Creation” dialog.
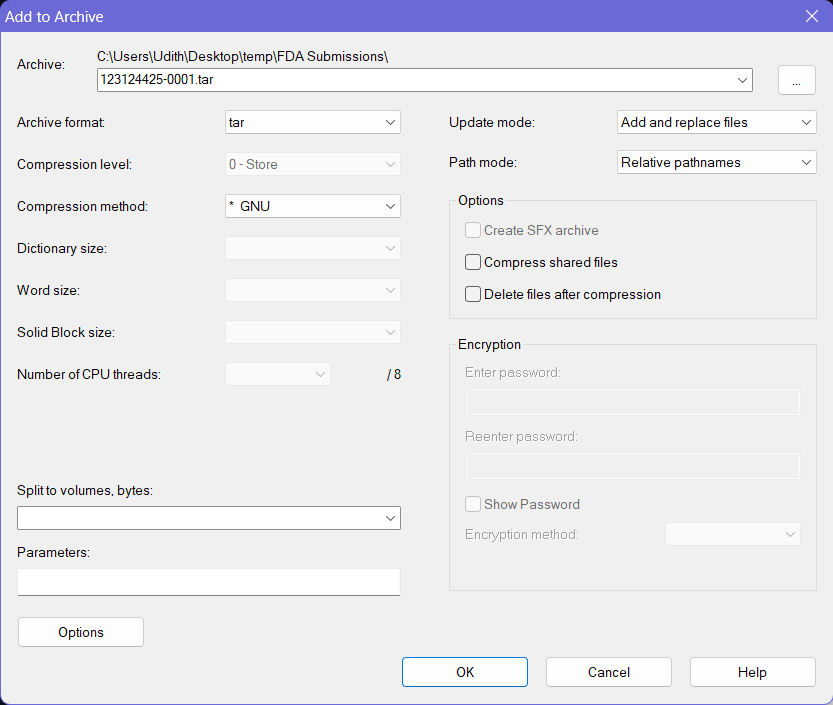
- Provide a proper name for the archive file following any established file naming conventions by your organization for the FDA submissions.
- Then select the Archive Format as tar, and Compression Method as * GNU. Keep the other options to the default values.
- Click OK button at the bottom and this will create a
.tarfile with the provided name at the same location where the folder you selected earlier was on.
GZipping the tar file
- Now select the previously created tar file through 7-Zip and click the Add button again (Do not select any other files or folders).
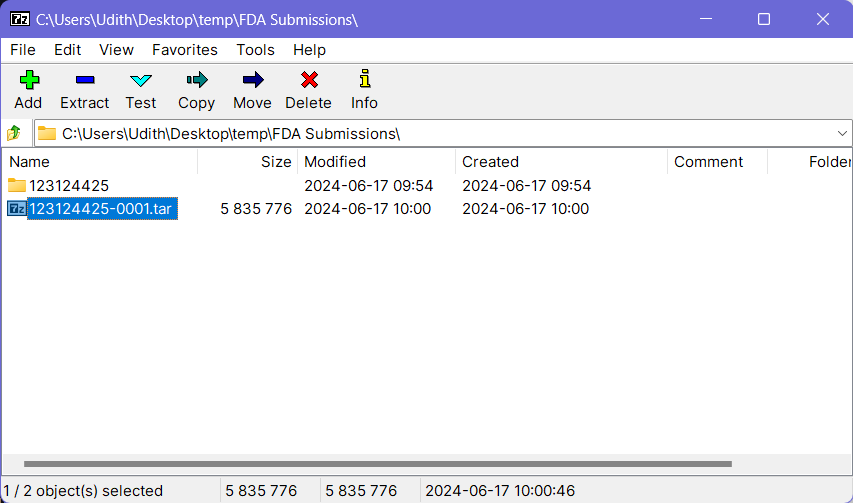
- Then keep the file name as it is and choose gzip as the Archive Format. Keep the other options to the default values.
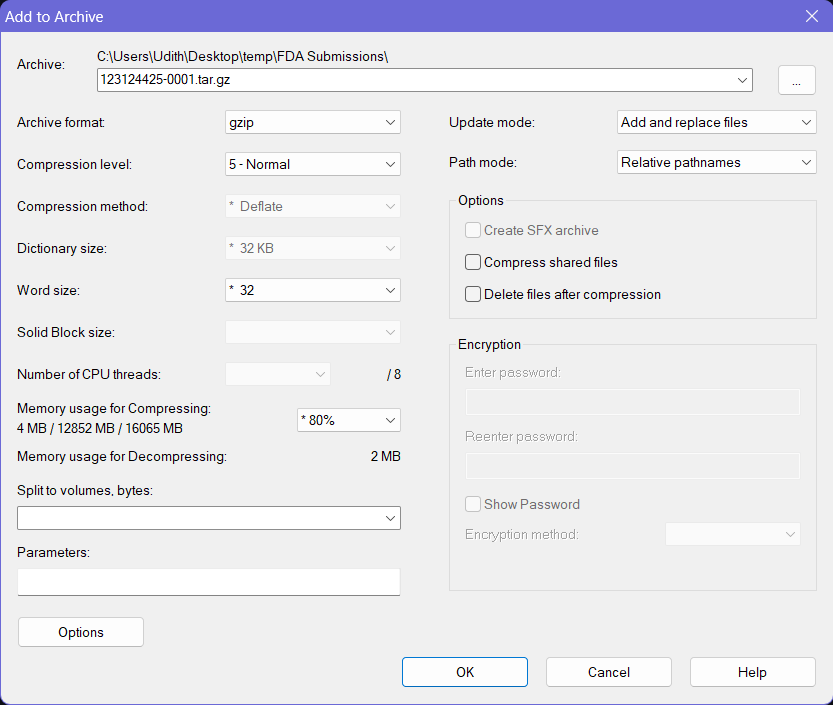
- Click OK button at the bottom and this will create a
.tar.gzfile with the provided name at the same location where the folder your source folder (and the.tarfile) was on. This file can be now submitted to the FDA using an AS2 connection via a software such as the MFT Gateway.
In Mac OS
At the time of writing this article, a comparable reliable GUI tool for macOS was unavailable. Therefore, we will utilize the well-known command line utility, GNU Tar, for this purpose on macOS.
Installing GNU Tar
We will be using Homebrew, a popular software package manager for macOS, to install the GNU Tar utility. If Homebrew is not already installed on your system, you can install it by executing the following command in the macOS terminal.
/bin/bash -c "$(curl -fsSL https://raw.githubusercontent.com/Homebrew/install/HEAD/install.sh)"
GNU Tar is available to be installed as a Homebrew Formulae for Mac. To
install it, you can open the terminal application and execute the following command.
brew install gnu-tar
You should see a line similar to the below, if the installation was successfully completed.
GNU "tar" has been installed as "gtar".
Creating the archive
To create the archive, first navigate to the folder where your submission-ready content is available, using the macOS
terminal.
cd /Users/john/Desktop/FDA-Submissions
Then, execute the following command to create a tar.gz archive that includes the submission folder and its contents.
gtar -cvzf 123124425-0002.tar.gz --format=gnu 123124425/
In the above command, 123124425-0002.tar.gz is the name of the archive to be created. You can replace it with an
appropriate name that adheres to your organization’s file naming conventions for FDA submissions. Additionally,
123124425/ is the directory to be included in the submission (generally this will be the one with Application ID as
the name), so ensure you replace this with the actual name of the directory on your computer.
Once the command is successfully executed, a tar.gz file with the specified name will be created in the current
folder. This file can be now submitted to the FDA using an AS2 connection via a software such as
the MFT Gateway.
The software recommendations provided in this article are based on general information and personal experience, and is offered for informational purposes only. We do not endorse or guarantee the performance, reliability, or suitability of the recommended software for any specific purpose. Before using any software, we strongly encourage you to perform your own research and due diligence to determine whether it meets your specific needs and requirements. Aayu Technologies does not endorse or assume responsibility for any software or third-party services mentioned.

Talk to an EDI Expert
Join hundreds of organizations already taking full control of their B2B AS2 communications with our trusted solutions. Contact us today to tailor a solution that fits your specific AS2 EDI needs.
Related Articles
View All BlogsExplore our product stack
Try before you buy with a 30-day Free Trial
No commitment, all value. Try the AS2 Solution Risk-Free and discover how our solutions can transform your business workflows. No credit card required.
Explore Your Possibilities
Elevate AS2 Communications with our EDI and AS2 Solutions
See how our AS2 and EDI solutions can simplify your integrations, boost efficiency, and keep you compliant—request a personalized demo today.