MFT Gateway is a hosted Software as a Service (SaaS) solution that enables file exchange over the AS2 or SFTP protocol, without the need to install or maintain.
- Blog
- ESG NextGen Submission Process | FDA Electronic Submissions
AS2
ESG NextGen Submission Process | FDA Electronic Submissions
Learn about ESG NextGen, the FDA’s modernized Electronic Submissions Gateway, and its secure, scalable submission methods - USP, AS2 and API.

Adheeb Shafik
Published: 04 Mar 2025

Table of Contents
The FDA Electronic Submissions Gateway (ESG) is a secure, high-performance portal for submitting regulatory documents to the FDA. It supports premarket and postmarket submissions, ensuring secure data exchange via industry-standard protocols and digital certificates. Acting as a single entry point, ESG automates processing, provides receipt acknowledgments, and routes submissions to the appropriate FDA offices.
ESG NextGen
ESG NextGen is the modernized version of the FDA Electronic Submissions Gateway (ESG), featuring a cloud-based architecture for improved performance, scalability, and security. It integrates an enterprise Identity and Access Management solution, enhances data submission bandwidth, and expands storage capacity while maintaining stable operation. ESG NextGen represents the next step in streamlining electronic regulatory submissions.
ESG NextGen Submission Methods
ESG NextGen will support regulatory submissions through three primary methods, offering enhanced flexibility and efficiency:
-
Unified Submission Portal (USP) – A web-based interface similar to the current ESG WebTrader method. It allows users to manually upload and submit regulatory documents through a secure online portal, making it a user-friendly option for organizations that do not require automated integration.
-
AS2 (Applicability Statement 2) – This method continues from the current ESG, enabling automated, secure, and reliable document exchange via the well-established AS2 protocol. It is ideal for organizations with existing AS2 infrastructure, ensuring seamless integration with FDA systems.
-
API (Application Programming Interface) – A new addition in ESG NextGen, the API method leverages RESTful web services to streamline submissions through automated, system-to-system communication. This method is expected to become the preferred submission approach in the future due to its scalability, efficiency, and ease of integration.
These submission methods ensure that ESG NextGen remains adaptable, secure, and ready for the evolving needs of regulatory submissions.
ESG NextGen Submission Process
USP
The Unified Submission Portal (USP) follows a submission process similar to ESG’s previous tool, WebTrader. The diagram below outlines the process in four key stages
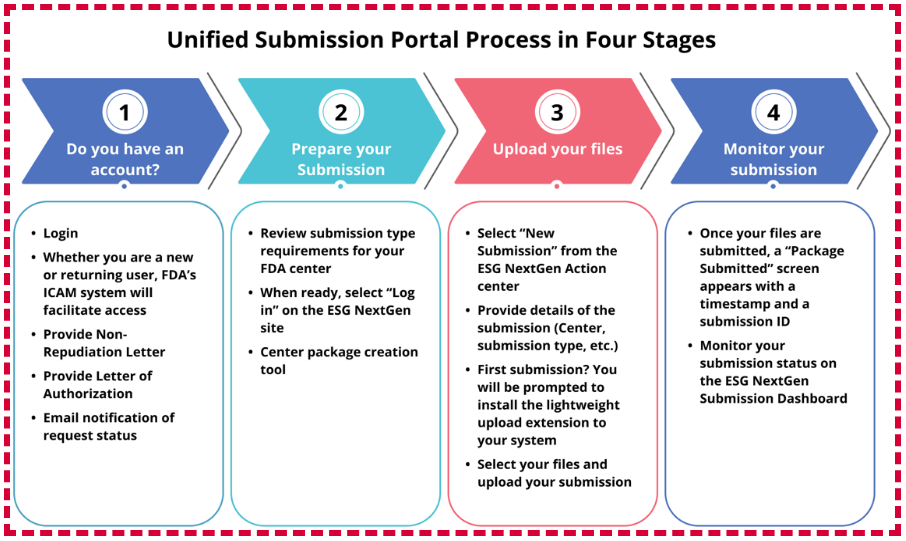
Source: FDA
AS2
The AS2 submission method allows users to utilize AS2-compliant software to submit documents to ESG NextGen. Submissions made via AS2 will also be accessible within the USP. However, AS2 is only available to users with an active AS2 account from the legacy ESG system.
API
ESG NextGen APIs simplify file submissions, status checks, and related tasks using RESTful standards for seamless integration. They provide secure data exchange through OAuth 2.0 authorization and ClientID-Secret authentication, granting access via validated tokens. For detailed API specifications, refer to the provided ESG NextGen API specifications.
All three submission methods in ESG NextGen offer users increased flexibility to meet their specific needs. Among these, the REST API is positioned as the long-term replacement for AS2. Although the timeline for decommissioning AS2 has not yet been determined, users can continue leveraging the AS2 method for submissions in the meantime.
Looking ahead, the AS2 Gateway will also incorporate support for the REST API, ensuring a smooth transition and improved capabilities for users. Stay tuned for future updates as ESG NextGen continues to evolve, making regulatory submissions more seamless and efficient.

Talk to an EDI Expert
Join hundreds of organizations already taking full control of their B2B AS2 communications with our trusted solutions. Contact us today to tailor a solution that fits your specific AS2 EDI needs.
Related Articles
View All BlogsExplore our product stack
Try before you buy with a 30-day Free Trial
No commitment, all value. Try the AS2 Solution Risk-Free and discover how our solutions can transform your business workflows. No credit card required.
Explore Your Possibilities
Elevate AS2 Communications with our EDI and AS2 Solutions
See how our AS2 and EDI solutions can simplify your integrations, boost efficiency, and keep you compliant—request a personalized demo today.