MFT Gateway is a hosted Software as a Service (SaaS) solution that enables file exchange over the AS2 or SFTP protocol, without the need to install or maintain.
Aayu Technologies LLC has recently released MFT Gateway 3.6.8 and the latest update supports submissions to the U.S. Food and Drug Administration (FDA). Let’s go through this article and see how FDA submissions work in MFT Gateway.
FDA Submissions
If you operate in the healthcare domain within US jurisdictions, here’s good news for you! MFT Gateway’s SaaS distribution is now supporting FDA submissions, allowing users to easily and securely connect to the Electronic Submissions Gateway (ESG) of US Food and Drug Administration (FDA) via AS2 protocol, for automated and high-volume regulatory submissions.
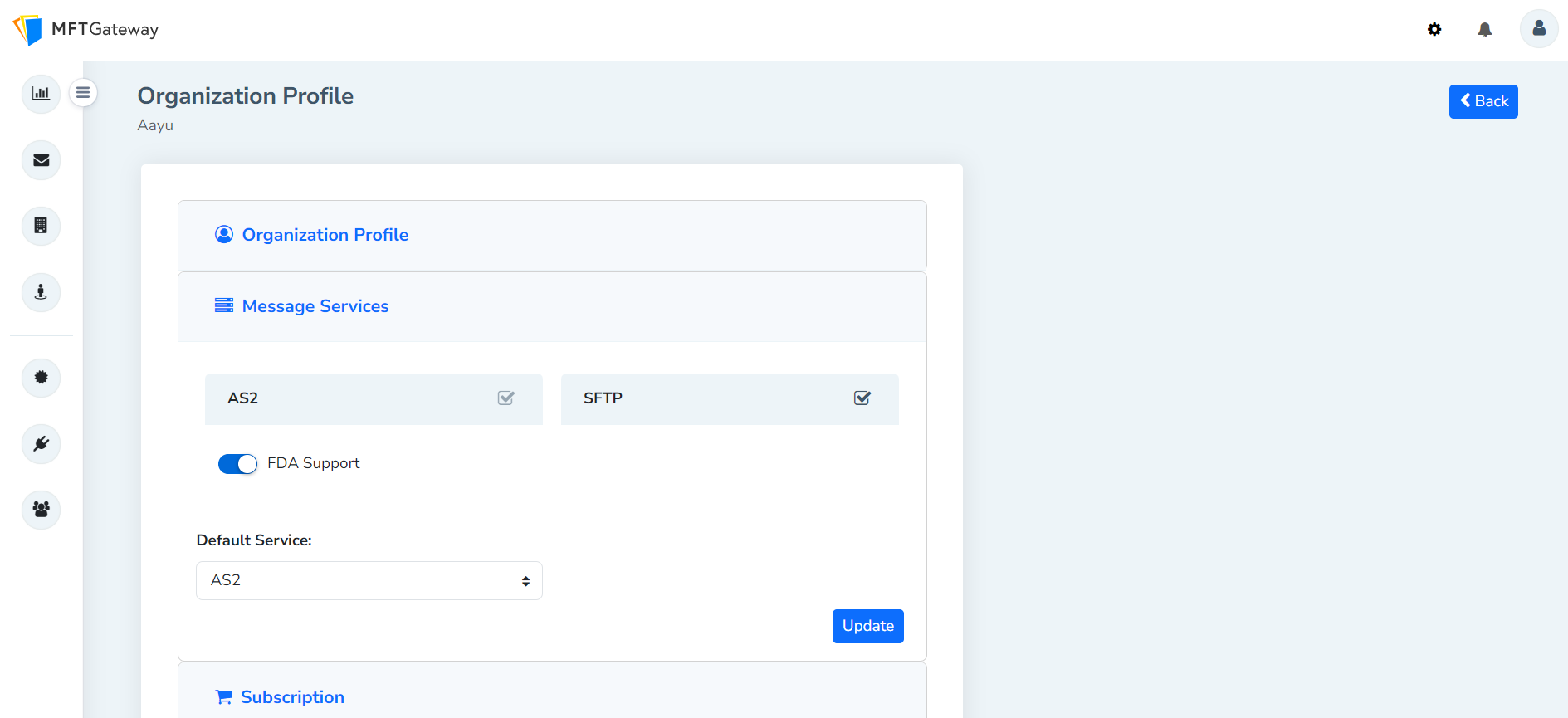
Users can enable this feature from the Message Services section under the Organization Profile.
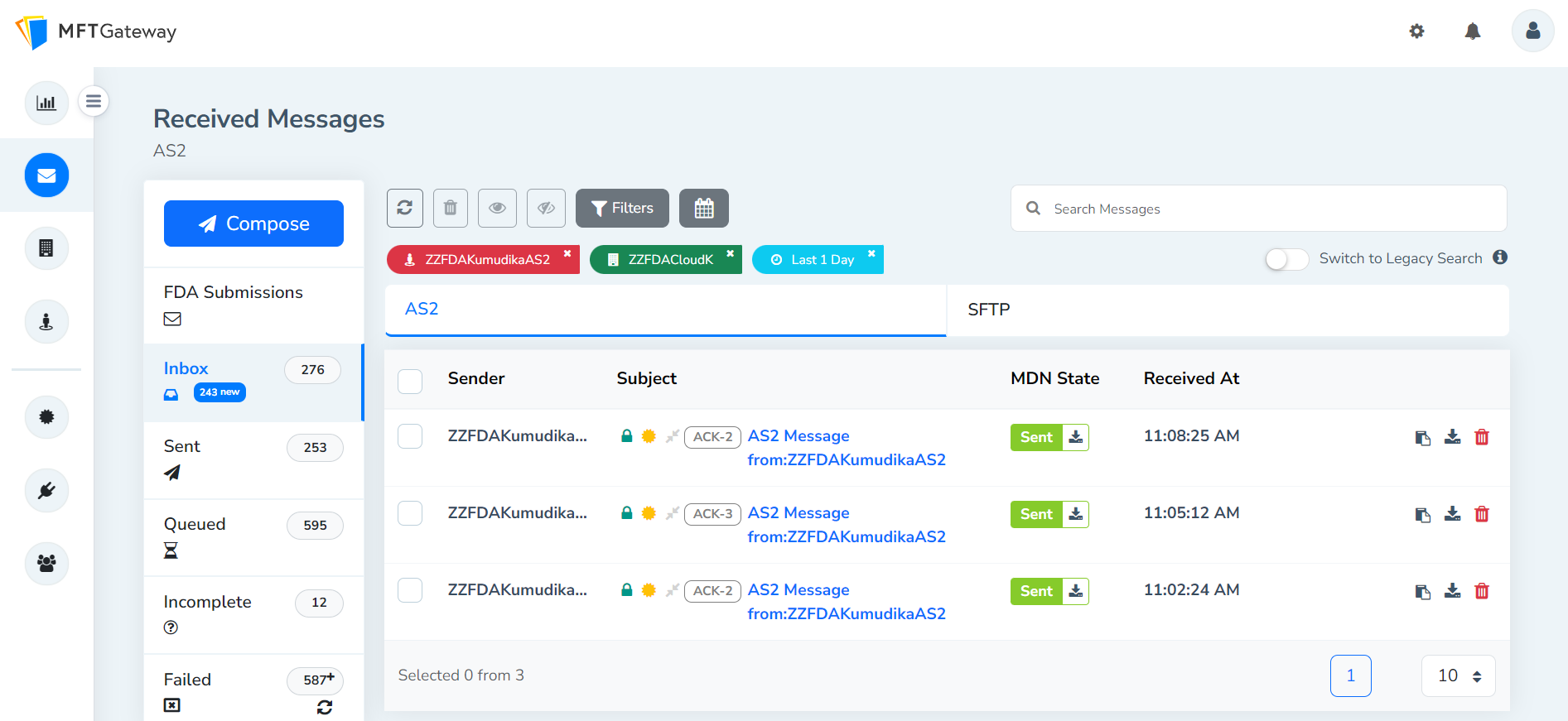
After the successful completion of AS2 message configurations, MFT Gateway users can perform FDA submissions and the submissions will be displayed under FDA Submissions as shown below.
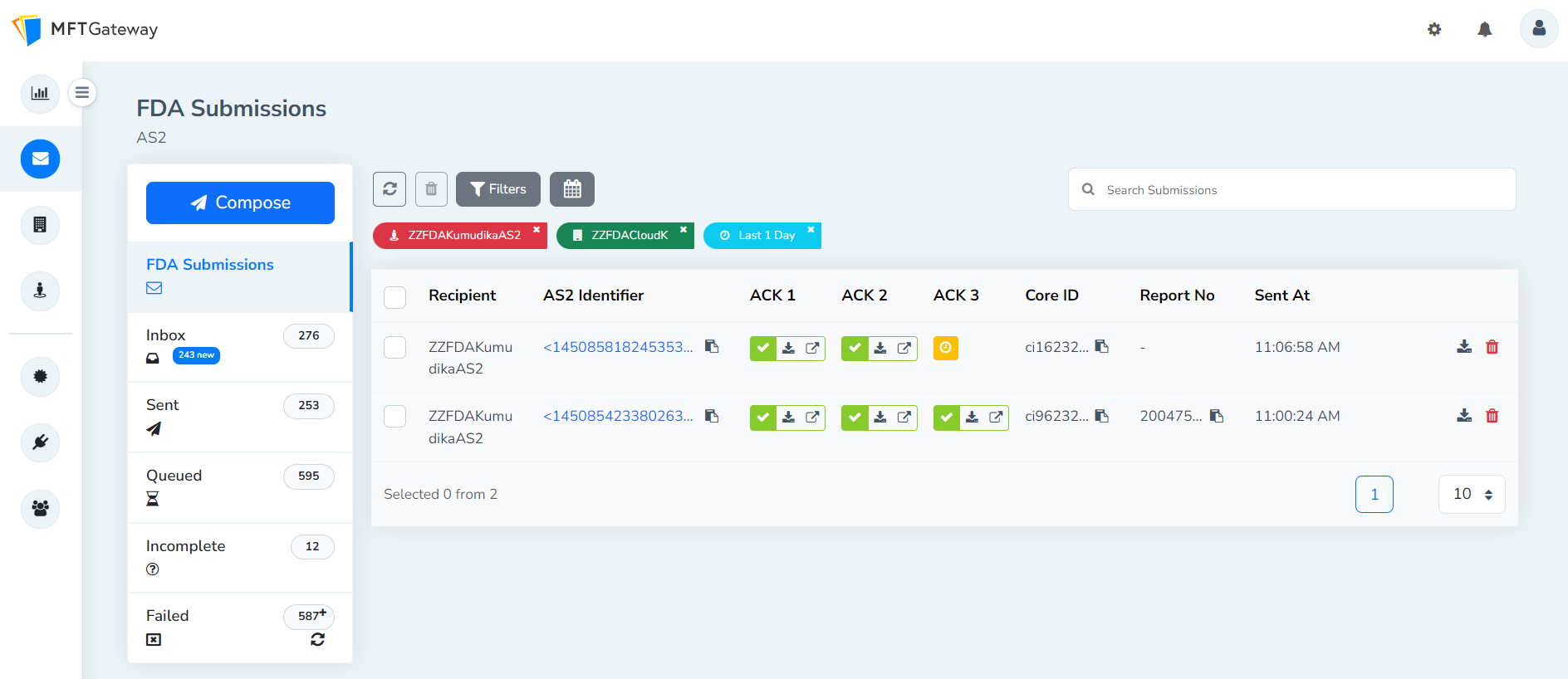
Once the successful acknowledgements for the submissions are received, the incoming messages will be marked with the corresponding acknowledgement tag as shown below for easy identification.
Conclusion
To summarize what’s new in MFT Gateway’s new release, the latest deployment supports FDA submissions allowing MFT Gateway users to easily connect to the Electronic Submissions Gateway (ESG) of the US Food and Drug Administration (FDA) via AS2 protocol. Give it a try and let us know what you think.
Sign Up for a 30 day Free Trial! Stay tuned for more updates!